Nature of Gas Particles...!!!
Understanding gas particles is crucial for grasping the physical properties and behavior of gases. From the kinetic molecular theory to the ideal gas law, these concepts explain the dynamic nature of gases and their significance in both natural and industrial contexts.
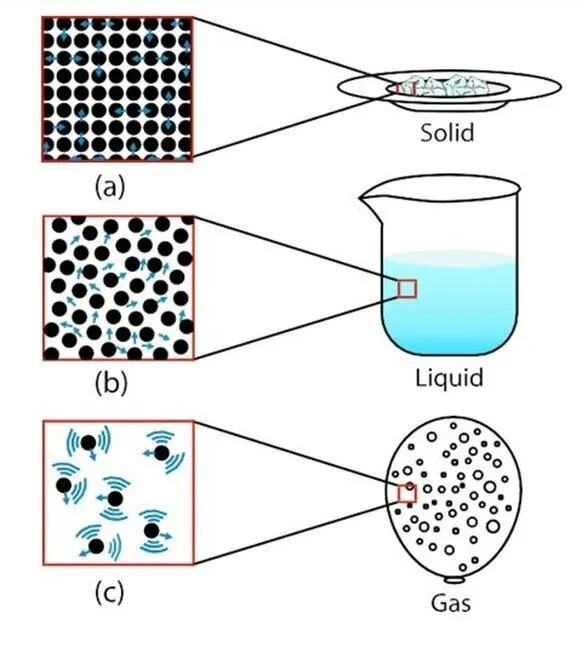
Composition and Structure
Gas particles can be atoms, molecules, or ions, depending on the type of gas. For instance, noble gases like helium (He) and neon (Ne) consist of individual atoms, while molecular gases like oxygen (O₂) and nitrogen (N₂) are composed of molecules. Some gases, such as carbon dioxide (CO₂) and water vapor (H₂O), are compounds made up of different elements.
Size and Mass
The size and mass of gas particles vary greatly. For example, a helium atom is much smaller and lighter than a carbon dioxide molecule. Despite these differences, gas particles are generally much smaller than those in solids and liquids, allowing them to move freely and fill any container they occupy.
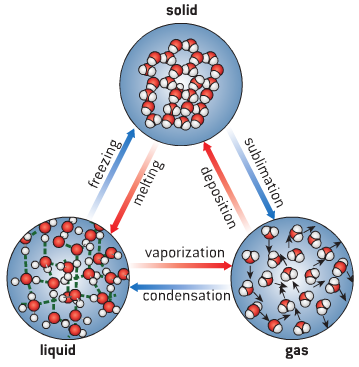
Behavior of Gas Particles
Kinetic Molecular Theory
The kinetic molecular theory describes the behavior of gas particles, stating that they are in constant, random motion. This theory explains several properties of gases:
-
Pressure: Gas pressure results from collisions between gas particles and the walls of their container. As particles move and collide, they exert force on the container's walls, creating pressure. More frequent and forceful collisions result in higher pressure.
-
Temperature: The temperature of a gas is directly related to the average kinetic energy of its particles. Higher temperatures mean particles move faster and have more energy.
-
Volume: Gases expand to fill the volume of their container. The volume occupied by a gas depends on the temperature, pressure, and number of particles.
-
Density: The density of a gas is influenced by its pressure, temperature, and the number of particles in a given volume.
Diffusion and Effusion
Gas particles exhibit diffusion and effusion due to their constant motion:
-
Diffusion: The process by which gas particles spread out to evenly distribute themselves within a container. This occurs because gas particles move randomly and collide with each other, eventually mixing uniformly.
-
Effusion: The escape of gas particles through a small hole in a container. According to Graham's law, lighter gas particles effuse faster than heavier ones.
Ideal Gas Law
The ideal gas law is a fundamental equation that describes the behavior of ideal gases. It is expressed as:
where is the pressure, is the volume, is the number of moles of gas, is the universal gas constant, and is the temperature in Kelvin. The ideal gas law assumes that gas particles have negligible volume and do not experience intermolecular forces, making it most accurate for gases at low pressures and high temperatures.
Real Gases
In reality, gas particles do have volume, and intermolecular forces do exist, especially at high pressures and low temperatures. Real gases deviate from ideal behavior under these conditions. The van der Waals equation is an adjusted version of the ideal gas law that accounts for these deviations:
Here, and are constants specific to each gas, representing intermolecular forces and the volume of gas particles, respectively.

Applications and Significance
Natural Processes
Gas particles play a vital role in numerous natural processes:
-
Atmospheric Gases: Oxygen, nitrogen, and carbon dioxide are essential for life on Earth. They support respiration, photosynthesis, and various biochemical cycles.
-
Weather and Climate: The movement and interactions of gas particles in the atmosphere drive weather patterns and influence climate.
Industrial Applications
Gas particles are critical in various industries:
-
Chemical Industry: Gases like hydrogen, oxygen, and nitrogen are used in chemical synthesis, refining, and manufacturing processes.
-
Energy Production: Natural gas, a mixture of hydrocarbons, is a major source of energy for electricity generation and heating.
Understanding gas particles is crucial for grasping the physical properties and behavior of gases. From the kinetic molecular theory to the ideal gas law, these concepts explain the dynamic nature of gases and their significance in both natural and industrial contexts. The study of gas particles remains a foundational aspect of chemistry and physics, with ongoing research uncovering new insights and applications.
What's Your Reaction?